What is Kinetic Energy of an Ejected Electron?
Definition: The kinetic energy of an ejected electron refers to the energy possessed by an electron that has been released or emitted from an atom or a material due to an external stimulus, such as a photon impact or thermal excitation. This energy is a result of the electron’s movement or motion. It is determined by its speed or velocity as it departs from the atom or material. The kinetic energy of the ejected electron can vary depending on several factors. These factors include the nature of the material, the energy of the incident photon, and the specific atomic interactions involved. In this article, I will solve examples of how to calculate the kinetic energy of an ejected electron from the metal.
The kinetic energy of an electron that escapes the surface of the metal does not rely on the incident light and it depends on the frequency.
Kinetic Energy of an Ejected Electron Formula
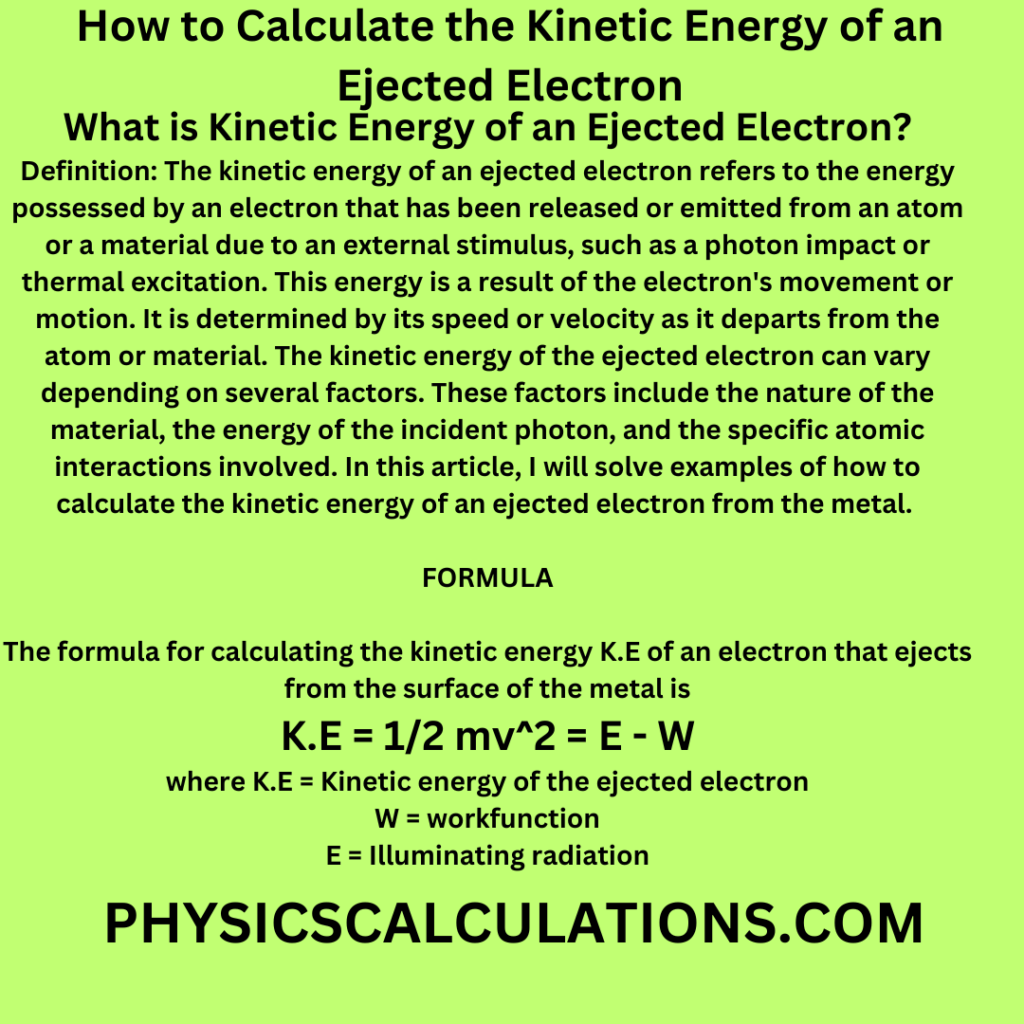
The formula for calculating the kinetic energy K.E of an electron that ejects from the surface of the metal is
K.E = 1/2 mv2 = E – W
where K.E = Kinetic energy of the ejected electron
W = workfunction
E = Illuminating radiation
You may also like to read:
How to Calculate Capacitive Reactance
Solved Problem: How to Calculate the Kinetic Energy of an Ejected Electron
Here are a few examples to help you understand how to calculate the kinetic energy of an ejected electron from the metal:
Problem
The work function of a metal is 4.65eV and the illumination of radiation on the metal is 6.86eV. What is the kinetic energy of the electrons that eject from the surface of the metal?
Solution
Data:
The work function of the metal, W = 4.65eV
Illuminated radiation, E = 6.86eV
eV = 1.6 x 10-19J
The kinetic energy of the electron, K.E =?
We can now apply the formula for the kinetic energy of the electron, which is
K.E = 1/2 mv2 = E – W
and we can write it as
K.E = E – W = 6.86eV – 4.65eV = 2.21eV = 2.21 x 1.6 x 10-19 = 35.36 x 10-20J
Therefore, the kinetic energy of the electron to escape from the surface of the metal is 35.36 x10-20 Joules
Table of Solved Problems
Sure, here’s a table with information for various elements, including their work function, ionization energy, the kinetic energy formula you provided, and the calculated kinetic energy based on the formula:
| Element | Work Function [W] (eV) | Ionization Energy [E] (eV) | Kinetic Energy Formula [K.E = E – W] | Calculated Kinetic Energy (eV) |
|---|---|---|---|---|
| Sodium | 2.28 | 5.14 | 5.14 – 2.28 | 2.86 |
| Copper | 4.65 | 7.73 | 7.73 – 4.65 | 3.08 |
| Gold | 5.1 | 9.22 | 9.22 – 5.1 | 4.12 |
| Silver | 4.74 | 7.58 | 7.58 – 4.74 | 2.84 |
| Zinc | 4.3 | 9.39 | 9.39 – 4.3 | 5.09 |
| Aluminum | 4.28 | 5.99 | 5.99 – 4.28 | 1.71 |
| Iron | 4.5 | 7.87 | 7.87 – 4.5 | 3.37 |
| Nickel | 5.01 | 7.64 | 7.64 – 5.01 | 2.63 |
| Titanium | 4.33 | 6.82 | 6.82 – 4.33 | 2.49 |
| Platinum | 5.65 | 9.0 | 9.0 – 5.65 | 3.35 |
I provided these values in the table for illustrative purposes on how to calculate the kinetic energy of an ejected electron. The kinetic energy formula provided is used to calculate the kinetic energy of ejected electrons based on the ionization energy of the illuminating radiation and the work function of the metal.
You may also like to read:
How to Calculate Power in a Circuit
How to Calculate the Wavelength of Light
Reference