What is Density?
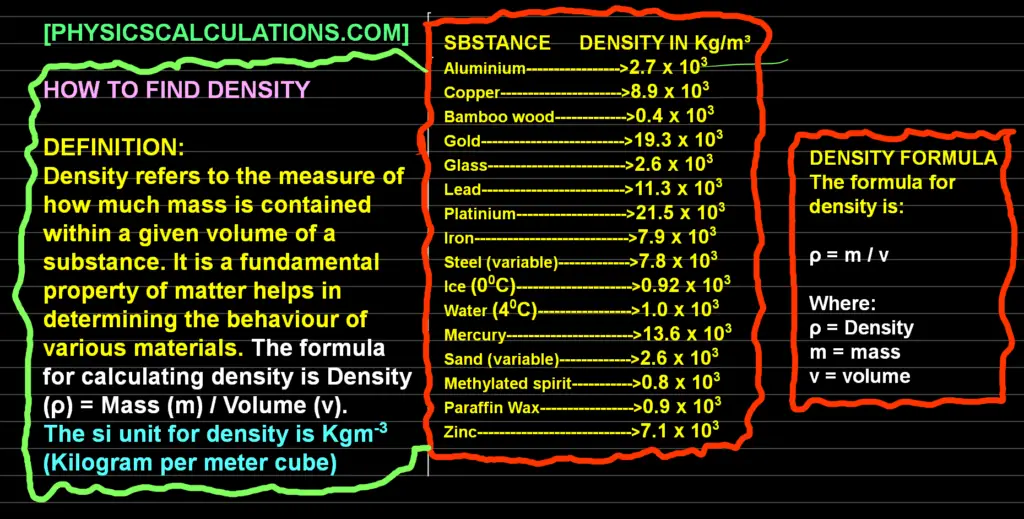
Definition of Density. Density refers to the measure of how much mass is contained within a given volume of a substance. It is a fundamental property of matter that helps in determining the behaviour of various materials. The formula for calculating density is Density (ρ) = Mass (m) / Volume (v)
Here is a simple table outlining the steps to find the density of an object or substance:
| Step | Description |
|---|---|
| First Step: | Identify the object or substance for which you want to find density. |
| Second Step: | Measure the mass (m) of the object or substance using a scale. |
| Third Step: | Measure the volume (V) of the object or substance using appropriate tools. |
| Fourth Step: | Use the formula [Density (ρ) = Mass (m) / Volume (V)] to calculate density. |
| Fifth Step: | Plug in the measured values of mass and volume into the formula. |
| Sixth Step: | Perform the division to find the density. Ensure consistent units, typically kilograms per cubic meter (kg/m³). |
| Seventh Step: | The result is the density of the object or substance. |
This table provides a comprehensive set of steps for finding the density of an object or substance. Following these steps ensures an organized and systematic approach to density calculations.
Density Formula
We can write the equation for density (density formula) mathematically as ρ = m / v. The si unit for density is Kgm-3 (Kilogram per meter cube).
In simpler terms, density tells us how tightly packed the particles of a substance are. If the particles are closely packed, the substance will have a higher density, while substances with loosely packed particles will have a lower density.
How to Find Density: Step by Step
Step 1: Gather the Required Information
To calculate density accurately, you need two pieces of information: the mass of the substance and its volume. Make sure you have a scale to measure mass and the necessary tools to determine volume.
Step 2: Measure the Mass
Using a scale or balance, measure the mass of the substance you want to find the density of. Ensure that your scale is properly calibrated for accurate measurements.
Step 3: Determine the Volume
The method for measuring volume depends on the shape of the object or substance. Here are a few common methods:
- Regular-Shaped Objects: For objects with regular shapes, such as cubes or rectangular prisms, you can use a ruler or calliper to measure the dimensions (length, width, and height) and calculate the volume accordingly. Multiply the three dimensions together to obtain the volume.
Volume = Length x Width x Height
2. Irregular-Shaped Objects: If the object has an irregular shape, you can use the water displacement method. Fill a graduated cylinder partially with water and record the initial volume. Carefully lower the object into the cylinder, by making sure you submerge it fully into the cylinder. The rise in water level represents the volume of the object.
3. Liquids: When dealing with liquids, you can directly measure the volume using a graduated cylinder. Ensure that the cylinder is on a flat surface and that your eye is level with the meniscus—the curved surface of the liquid.
Step 4: Calculate the Density
Once you have measured the mass and determined the volume, you can calculate the density using the formula:
Density = Mass / Volume or ρ = m / v
Divide the mass by the volume to obtain the density of the substance. Make sure the units are consistent throughout the calculation. For example, if the mass is in grams and the volume is in cubic centimetres, the density will be in grams per cubic centimetre (g/cm³).
How to Find Density: Solved Problem
Assuming we have a substance of mass 4 x 105 kg and the volume of the substance is 1000 m³. We can easily find the density by saying:
ρ = m / v = (400000 kg) / (1000 m³) = 0.4 x 103 Kg/m³.
Therefore, the above substance is bamboo wood which has a density of 0.4 Kg/m³
Table of Density of Substances
| Substance | Density at Kg/m³ |
| Aluminium | 2.7 x 103 |
| Copper | 8.9 x 103 |
| Bamboo wood | 0.4 x 103 |
| Gold | 19.3 x 103 |
| Glass | 2.6 x 103 |
| Lead | 11.3 x 103 |
| Platinium | 21.5 x 103 |
| Iron | 7.9 x 103 |
| Steel (variable) | 7.8 x 103 |
| Ice (00C) | 0.92 x 103 |
| Water (40C) | 1.0 x 103 |
| Mercury | 13.6 x 103 |
| Sand (Variable) | 2.6 x 103 |
| Methylated Spirit | 0.8 x 103 |
| Paraffin wax | 0.9 x 103 |
| Zinc | 7.1 x 103 |
Applications of Density Calculation
The calculation of density finds applications in various fields and industries. Here are some notable examples:
1. Material Identification
Determining the density of a substance can help identify the material. Different substances have unique densities due to variations in their atomic or molecular composition. By comparing the calculated density with known values, scientists and analysts can identify unknown materials.
2. Quality Control
Density measurement is very important in quality control processes for industries such as pharmaceuticals, food, and manufacturing. With diligent monitoring of the density of products, manufacturers can ensure consistency and detect any deviations that may indicate production issues.
3. Buoyancy
Density helps in determining the buoyancy of objects in fluids. Archimedes’ principle states that an object submerged in a fluid experiences an upward buoyant force equal to the weight of the displaced fluid. When we compare the density of an object with the density of the fluid, we can determine if the object will float or sink.
4. Earth Sciences
Geologists use density to identify and classify rocks and minerals. Additionally, density variations in the Earth’s interior help in understanding the planet’s structure, including the composition of the core, mantle, and crust.
Frequently Asked Questions
1: What are some common units of density?
1: Density can be in various units depending on the substance and the context. The most common unit for density is Kg/m³. Other common units include grams per cubic centimetre (g/cm³), kilograms per litre (kg/L), and pounds per cubic inch (lb/in³).
2: Can density be negative?
2: No, density cannot be negative. Density is a measure of mass per unit volume, and since both mass and volume are positive quantities, density will always be positive or zero.
3: Can the density of a substance change?
3: The density of a substance remains constant under normal conditions, assuming the temperature and pressure remain unchanged. However, extreme conditions, such as high temperatures or pressures, can cause changes in density.
4: Is density the same as weight?
4: No, density and weight are different. Weight refers to the force exerted on an object due to gravity, while density is the measure of how much mass is contained within a given volume. Weight depends on the gravitational field strength, while density is an intrinsic property of the substance.
5: How is density used in real life?
5: Density has numerous practical applications. It is used in fields such as engineering, chemistry, and geology. Density measurements are essential for determining the purity of substances, identifying materials, designing structures, and even in everyday tasks like cooking.
6: Can density help identify substances?
6: Yes, density plays a significant role in identifying substances. Each material has a unique density, allowing scientists and researchers to identify unknown substances by comparing their density with known values.
Conclusion
In conclusion, the knowledge of how to find density will help you in various scientific disciplines and everyday scenarios. Thus, by following the step-by-step guide provided in this article, you can calculate the density of different substances accurately. Remember, density is a fundamental property of matter that helps us understand the behaviour and characteristics of materials around us.
You may also like to read: