What is the Particle Nature of Matter?
Definition
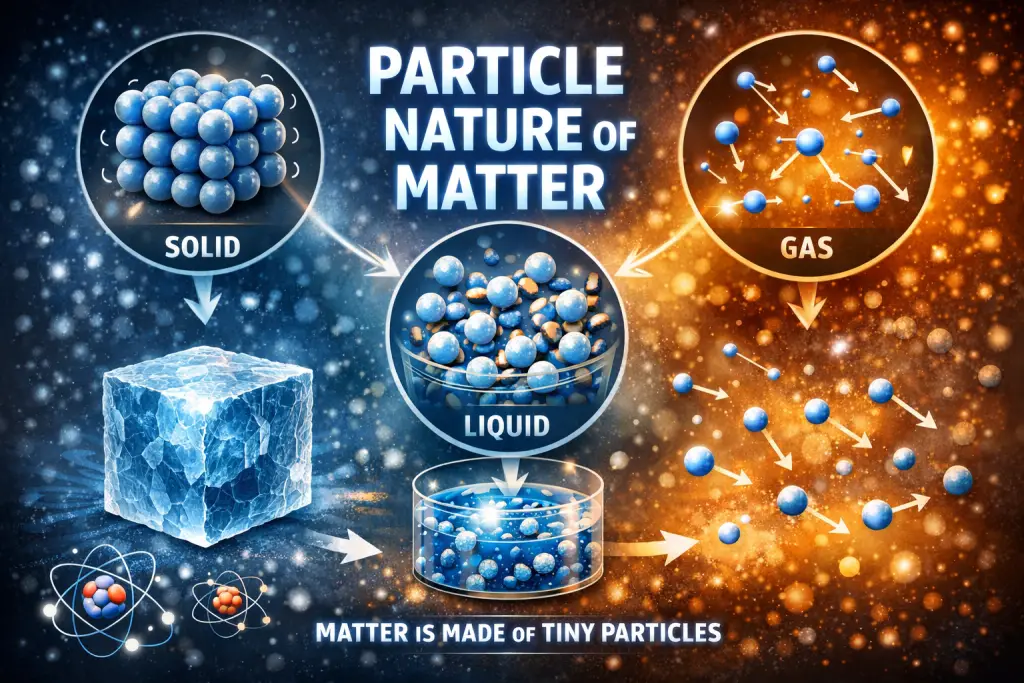
The particle nature of matter states that all matter is made up of tiny, discrete particles such as atoms and molecules.
Explanation
According to this concept, matter is not continuous but consists of very small particles that are constantly in motion. These particles have spaces between them and attract one another with forces of attraction. The arrangement and movement of these particles determine whether a substance exists as a solid, liquid, or gas.
Imagine
Imagine a classroom filled with students 👩🏫. If they sit very close and hardly move, it is like a solid. If they can move around but stay close, it is like a liquid. If they spread out and move freely, it is like a gas. This is how particles behave in different states of matter.
In simple terms
Matter is made up of tiny particles that are always moving.
Formula
There is no single formula for the particle nature of matter, but it is explained by the kinetic theory of matter.
Key Points
- Matter is made up of atoms and molecules
- Particles are extremely small
- There are spaces between particles
- Particles are always in motion
- There are forces of attraction between particles
Examples
- Smell of perfume spreading in a room
- Dissolving sugar in water
- Diffusion of gases
- Evaporation of water
Applications
- 🧪 Understanding chemical reactions
- 🌡️ Explaining changes of state
- 🏭 Industrial gas production
- 🫁 Understanding respiration
- 🧊 Refrigeration systems
Question
How does the spreading of perfume in a room support the particle nature of matter?
Answer
The spreading of perfume shows that gas particles move randomly and freely, filling the available space.
Solved Problem
Question:
A drop of ink spreads uniformly in a beaker of water without stirring. What does this demonstrate?
Answer:
It demonstrates that particles of matter are in constant motion and there are spaces between them, allowing diffusion to occur.