The 3 Laws of Thermodynamics
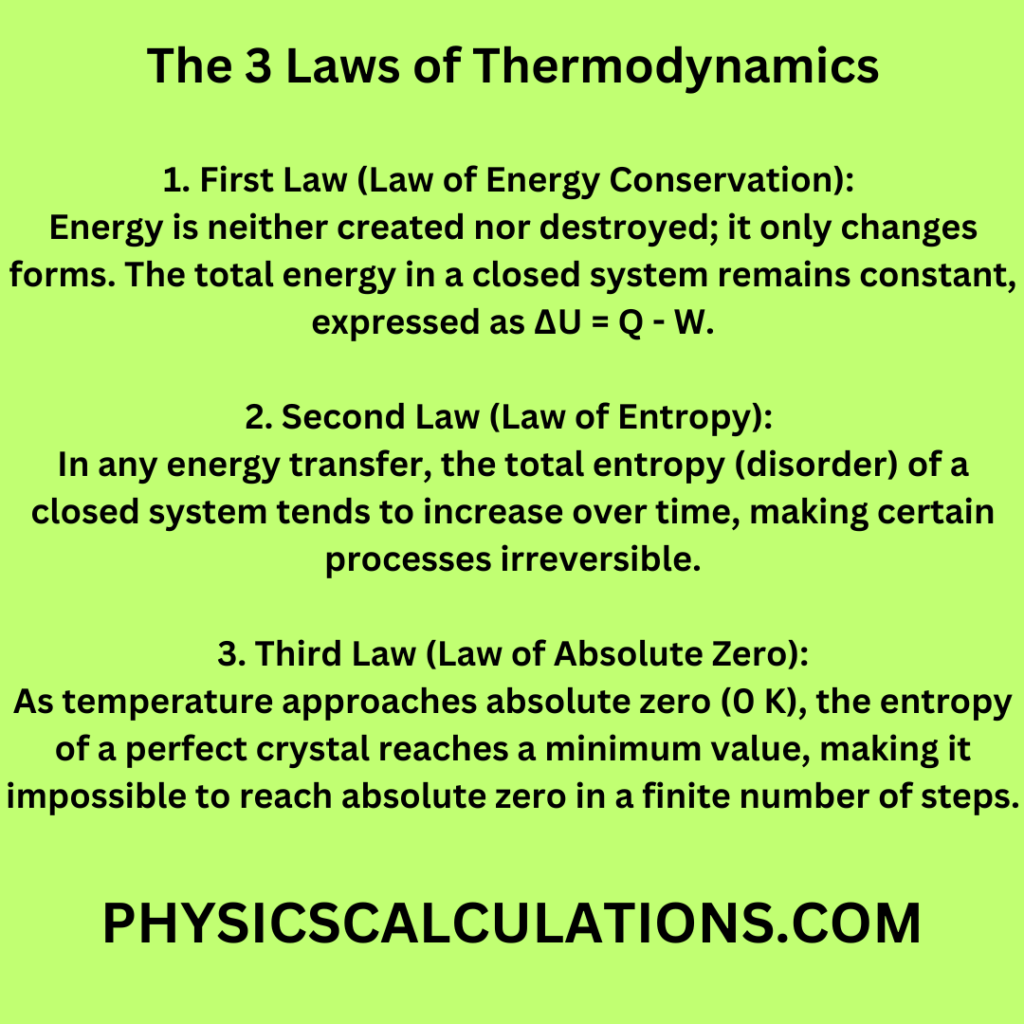
In thermodynamics, there exist three fundamental laws that govern the behaviour of energy and its transformation within closed systems. Here is a simple table outlining the three laws of thermodynamics:
| Law | Description |
|---|---|
| First Law (Law of Energy Conservation) | Energy cannot be created or destroyed; it can only change forms. The total energy in a closed system remains constant. Mathematically, ΔU = Q – W, where (ΔU) is the change in internal energy, (Q) is heat added to the system, and (W) is work done by the system. |
| Second Law (Law of Entropy) | In any energy transfer or transformation, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state. Entropy, a measure of disorder, tends to increase over time. |
| Third Law (Law of Absolute Zero) | As temperature approaches absolute zero (0 K), the entropy of a perfect crystal approaches a minimum value. This implies that it is impossible to reach absolute zero in a finite number of steps. |
This table provides a clear overview of the three laws of thermodynamics, explaining the fundamental principles that govern the behaviour of energy and systems in physics.
1. The First Law of Thermodynamics: Conservation of Energy
1.1 What is the First Law of Thermodynamics?
The First Law of Thermodynamics, also known as the Law of Energy Conservation states that energy cannot be created or destroyed within an isolated system. It can only be converted from one form to another or transferred between different parts of the system. In essence, the total energy of a closed system remains constant.
1.2 Understanding the First Law of Thermodynamics
To grasp the significance of the First Law, we must recognize that energy is an invaluable resource that persists in various forms. These forms include: heat, mechanical work, or electromagnetic radiation. This law reminds us that while energy can change its form, its total amount in an isolated system remains fixed.
An example that highlights the First Law of Thermodynamics is the operation of a heat engine. When fuel is burned, chemical energy is converted into thermal energy. It will in turn transform into mechanical work to propel the engine. The total energy input and output of the system remain balanced, demonstrating the principle of energy conservation.
1.3 Applications of the First Law of Thermodynamics
The First Law of Thermodynamics finds applications in numerous scientific and engineering fields. It serves as the foundation for studying heat transfer, energy conversion processes, and even the behaviour of living organisms. From understanding the inner workings of power plants to analyzing the energy flow within ecosystems. This law provides invaluable insights into the dynamics of energy transformation.
2. The Second Law of Thermodynamics: Entropy and Disorder
2.1 What is the Second Law of Thermodynamics?
The Second Law of Thermodynamics introduces the concept of entropy, which measures the degree of disorder or randomness within a system. It states that the entropy of an isolated system tends to increase over time. It leads to an inevitable progression toward equilibrium.
2.2 Unraveling the Second Law of Thermodynamics
To grasp the essence of the Second Law, we must comprehend the concept of entropy. Entropy is a measure of the system’s microscopic arrangements or the number of possible states it can occupy. The Second Law reveals that in the absence of external intervention, the system’s entropy tends to increase, resulting in a higher degree of disorder.
The common observation with this law is that hot objects cool down over time. For example, a cup of coffee left untouched eventually reaches room temperature. In both cases, the energy transfers from the hotter object to the cooler surroundings. This will eventually lead to an increase in entropy and a shift towards equilibrium.
2.3 Implications and Applications of the Second Law of Thermodynamics
The Second Law of Thermodynamics has far-reaching implications. It explains why certain processes, such as heat transfer, only occur in specific directions and not in reverse. It also sheds light on the limitations of energy conversion devices. These devices are heat engines, by introducing the concept of thermal efficiency.
This law plays a vital role in fields like chemistry, biology, and environmental science. It helps us understand chemical reactions, the behaviour of complex biological systems, and the irreversibility of natural processes. By studying the Second Law, scientists can devise strategies to enhance energy efficiency. They can also minimize waste, and make informed decisions regarding sustainable practices.
3. The Third Law of Thermodynamics: Absolute Zero and Perfect Crystals
3.1 What is the Third Law of Thermodynamics?
The Third Law of Thermodynamics states that as the temperature of a system approaches absolute zero (0 Kelvin or -273.15 degrees Celsius), the entropy of the system approaches a minimum or a constant value. This law provides insights into the behavior of matter at extremely low temperatures.
3.2 Unveiling the Third Law of Thermodynamics
The Third Law brings us to the intriguing concept of absolute zero, the hypothetical temperature at which all molecular motion ceases. As the temperature of a system nears absolute zero, the disorder and randomness within the system decrease, and its entropy reaches a minimum value.
This law finds significance in the study of condensed matter physics, particularly in exploring phenomena like superconductivity and superfluidity that manifest at ultralow temperatures. The Third Law provides a foundation for understanding the behaviour of matter and the transitions it undergoes as the temperature approaches absolute zero.
3.3 Realizing the Implications of the Third Law of Thermodynamics
The Third Law of Thermodynamics has profound implications for researchers and scientists investigating low-temperature phenomena. It sets a benchmark for the behaviour of materials at extreme conditions and helps us comprehend the limits of achievable cooling. By understanding the Third Law, scientists can explore exotic states of matter and develop novel technologies that harness the properties of materials at ultralow temperatures.
FAQs (Frequently Asked Questions)
FAQ 1: What are the key principles of the 3 laws of thermodynamics?
The 3 laws of thermodynamics comprise the principles that govern energy conservation, entropy, and the behaviour of matter at extreme temperatures. The First Law refers to the conservation of energy within an isolated system. The Second Law introduces the concept of entropy and explains the directionality of natural processes. The Third Law relates to absolute zero and the behaviour of matter at extremely low temperatures.
FAQ 2: How do the laws of thermodynamics impact everyday life?
The laws of thermodynamics have a profound impact on our everyday lives. From the energy efficiency of household appliances to the behaviour of engines and the design of efficient industrial processes, the laws of thermodynamics underpin many aspects of our technological advancements and environmental considerations.
FAQ 3: Are the laws of thermodynamics universally applicable?
Yes, the laws of thermodynamics are universally applicable. They govern the behaviour of energy and matter within closed systems, irrespective of their scale or composition. These laws are fundamental to our understanding of the physical world and we employ them across various scientific disciplines.
FAQ 4: Can the laws of thermodynamics be violated?
The laws of thermodynamics are fundamental principles and have been extensively tested and validated through countless experiments and observations. While there may be situations where it seems like these laws are being violated, further analysis usually reveals that other factors are at play, and the laws of thermodynamics remain intact.
FAQ 5: How do the laws of thermodynamics relate to the field of engineering?
The laws of thermodynamics form the backbone of engineering disciplines. They are used to design efficient energy systems, optimize processes, and ensure the reliability of mechanical devices. Engineers rely on the principles of thermodynamics to develop sustainable solutions, improve energy efficiency, and address complex challenges in various engineering domains.
FAQ 6: What are some practical applications of the laws of thermodynamics?
The laws of
thermodynamics find applications in numerous fields, including power generation, chemical engineering, materials science, environmental science, and even biology. From designing more efficient engines and optimizing manufacturing processes to understanding climate systems and studying the behavior of living organisms, the laws of thermodynamics provide a robust framework for scientific exploration and technological advancements.
Conclusion
In conclusion, the 3 laws of thermodynamics provide a solid foundation for understanding the behavior of energy, entropy, and matter within closed systems. The First Law emphasizes the conservation of energy, the Second Law reveals the progression of entropy and disorder, and the Third Law uncovers the behavior of matter at ultralow temperatures. These laws shape our understanding of the physical world and find applications in a wide range of scientific disciplines and technological advancements. By delving into the intricacies of thermodynamics, we gain valuable insights that propel us toward a more sustainable and efficient future.
You may also like to read: