1. What is the Shell Model of Atom?
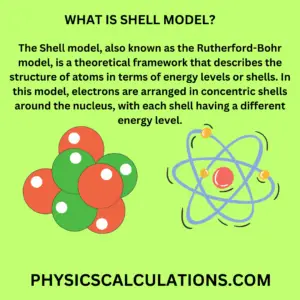
The Shell model of atom, also known as the Rutherford-Bohr model, is a theoretical framework that describes the structure of atoms in terms of energy levels or shells. In this model, electrons are arranged in concentric shells around the nucleus, with each shell having a different energy level.
This model of atom is a fundamental concept in atomic physics that explains the structure of atoms. It is a powerful tool that has helped scientists understand the behaviour of electrons and their interaction with the nucleus. In this article, we will delve into the Shell model, its history, and how it has transformed our understanding of the atomic structure.
2. The History of the Shell Model
The Shell model was first proposed by Niels Bohr in 1913, as an improvement to the Rutherford model. The Rutherford model suggested that electrons orbit the nucleus in a manner similar to planets orbiting the sun. However, Bohr realized that the Rutherford model could not explain the emission spectra of hydrogen. He postulated that electrons can only occupy certain discrete energy levels and that the energy absorbed or emitted during transitions between these levels is quantized. This was a groundbreaking idea at the time, as it provided a theoretical framework for understanding the spectral lines of atoms.
3. How does the Shell Model work?
In the Shell model, electrons are arranged in shells based on their energy levels. The shell closest to the nucleus has the lowest energy level, while the outermost shell has the highest energy level.
Each shell has a maximum number of electrons it can hold. The first shell can hold up to 2 electrons, the second shell can hold up to 8 electrons, the third shell can hold up to 18 electrons, and so on.
The number of electrons in the outermost shell is called the valence electrons. These electrons are responsible for the chemical properties of an atom.
4. The Pauli Exclusion Principle
Central to the Nuclear Shell Model is the Pauli Exclusion Principle, a fundamental concept in quantum mechanics. Enunciated by Wolfgang Pauli, this principle asserts that no two fermions, such as protons and neutrons, can occupy the same quantum state simultaneously. This principle serves as the guiding force behind the organization of particles within the nuclear shells.
5. Energy Levels and Shell Structure
Energy levels play a pivotal role in the Nuclear Shell Model, dictating the arrangement of protons and neutrons within the atomic nucleus. These particles form distinct shells analogous to the electron shells in the Bohr Atomic Model. The filling of these nuclear shells follows a pattern that reflects the intricate dance of particles, akin to the orbits of electrons.
6. Applications of the Shell Model
The utility of the Nuclear Shell Model extends beyond theoretical frameworks. Its predictive power allows scientists to anticipate nuclear properties, including angular momentum and nuclear spin. Furthermore, the model adapts to highly unstable nuclei, paving the way for alternative models like the Collective Model and the Liquid-drop Model.
7. Atomic Shell Model and Electron Arrangement
Drawing parallels between the Nuclear Shell Model and the Atomic Shell Model provides a holistic understanding of the particle arrangement in both atomic and nuclear systems. The electron arrangement around the nucleus mirrors the filling of energy levels in the nuclear shells. Exploring the intricacies of the Atomic Shell Model enhances our comprehension of electron behaviour in atoms.
8. Magic Numbers in Nuclear Structure
Magic numbers, a concept integral to the Nuclear Shell Model, profoundly influence the stability of nuclei. Nuclei with magic numbers of protons and neutrons exhibit unique stability, akin to the special stability observed in the noble gases of the periodic table. Understanding these magic numbers is vital for revealing the secrets of nuclear structure.
9. Magic Numbers and Stability
The concept of “magic numbers” emerges as a key aspect of the Nuclear Shell Model, defining certain neutron and proton configurations that endow nuclei with exceptional stability. These magic numbers, such as 2, 8, 20, 28, 50, and 82, represent complete nuclear shells. Nuclei with magic numbers of protons and neutrons exhibit enhanced stability, resembling the noble gases in the periodic table.
10. The Advantages of the Shell Model
The Shell model has revolutionized our understanding of the atomic structure. It has provided a way to explain the behaviour of electrons and their interactions with the nucleus. Some of the advantages of the Shell model include:
- It provides a framework for understanding the spectral lines of atoms.
- It helps explain the chemical properties of elements.
- It has led to the development of new technologies, such as X-ray crystallography and electron microscopy.
11. The Limitations of the Shell Model
While the Shell model has been incredibly useful in atomic physics, it is not without limitations. The Shell Model, while effective in explaining nuclear structure, has limitations. It struggles to predict nuclear properties in heavier elements and doesn’t fully capture certain nuclear phenomena, such as collective motion and high-spin states. Additionally, it doesn’t account for the intricate dynamics of nucleon-nucleon interactions in all situations.
12. The Uncertainty Principle
The Shell model assumes that electrons can be treated as particles with fixed positions and momenta. However, this assumption is not entirely accurate. According to the Uncertainty Principle, it is impossible to precisely determine both the position and momentum of an electron.
13. The Failure to Explain Multi-electron Atoms
The Shell model works well for single-electron atoms such as hydrogen. However, it fails to explain the behaviour of multi-electron atoms. These atoms have more complex electron configurations, and the interactions between electrons can lead to deviations from the Shell model predictions.
14. Conclusion
The Shell model is a crucial concept in atomic physics. It provides a framework for understanding the structure of atoms and has led to numerous technological advancements. While the model has limitations, it remains a powerful tool for scientists studying the behaviour of electrons.
15. Frequently Asked Questions (FAQs)
- What is the difference between the Shell model and the Rutherford model? The Rutherford model suggests that electrons orbit the nucleus in a manner similar to planets orbiting the sun. The Shell model, on the other hand, describes the structure of atoms in terms of energy levels or shells.
- What is the maximum number of electrons each shell can hold? The first shell can hold up to 2 electrons, the second shell can hold up to 8 electrons, the third shell can hold up to 18 electrons, and so on.
- What are valence electrons? Valence electrons are the electrons in the outermost shell of an atom. These electrons are responsible for the chemical properties of an atom.
- What is the Uncertainty Principle? The Uncertainty Principle states that it is impossible to precisely determine both the position and momentum of a particle, such as an electron.
- What are some of the limitations of the Shell model? The Shell model assumes that electrons can be treated as particles with fixed positions and momenta, which is not entirely accurate according to the Uncertainty Principle. The model also fails to explain the behaviour of multi-electron atoms.
You may also like to read:
Biological Effects of Radiation: Understanding the Risks