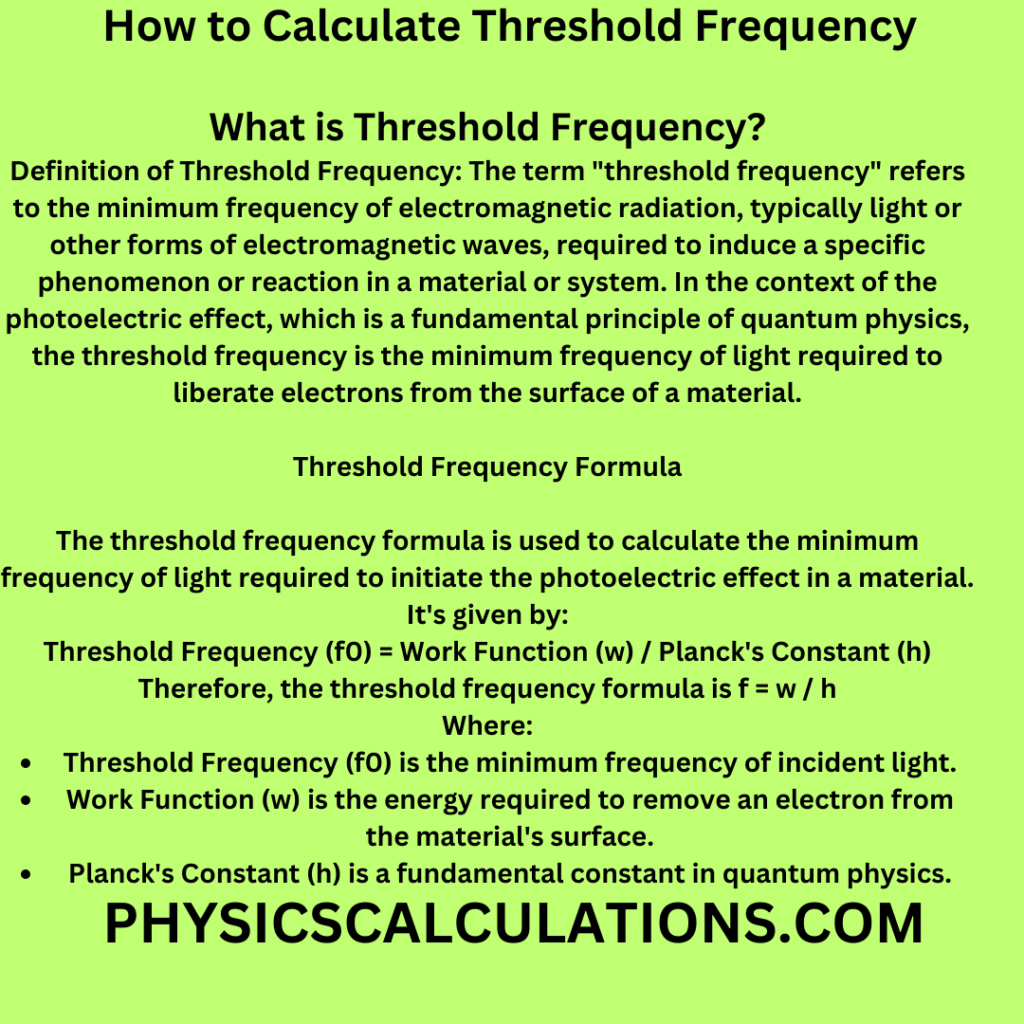
What is Threshold Frequency?
Definition of Threshold Frequency: The term “threshold frequency” refers to the minimum frequency of electromagnetic radiation, typically light or other forms of electromagnetic waves, required to induce a specific phenomenon or reaction in a material or system. In the context of the photoelectric effect, which is a fundamental principle of quantum physics, the threshold frequency is the minimum frequency of light required to liberate electrons from the surface of a material. In this article, you will learn how to calculate threshold frequency.
When light of a frequency equal to or greater than the threshold frequency strikes the material’s surface, it imparts enough energy to the electrons on the surface to overcome the binding forces that hold them in place. As a result, these electrons are emitted from the material, creating what is known as the photoelectric effect. Frequencies of light below the threshold frequency, however intense, do not possess sufficient energy to dislodge electrons from the material.
The concept of threshold frequency is integral to understanding how light interacts with matter on a microscopic scale and plays a significant role in the study of quantum mechanics and the behavior of subatomic particles.
Threshold Frequency Formula
The threshold frequency formula is used to calculate the minimum frequency of light required to initiate the photoelectric effect in a material. It’s given by:
Threshold Frequency (f0) = Work Function (w) / Planck’s Constant (h)
Therefore, the threshold frequency formula is f0 = w / h
Where:
- Threshold Frequency (f0) is the minimum frequency of incident light.
- Work Function (w) is the energy required to remove an electron from the material’s surface.
- Planck’s Constant (h) is a fundamental constant in quantum physics.
In equation form: f0 = w / h
Step-by-Step Guide: How to Calculate Threshold Frequency
Here is a comprehensive guide on how to calculate the threshold frequency of quantum energy:
- To calculate threshold frequency, we need to read and understand the question.
2. Read the question for the first time.
3. Try reading the question again, but this time underline any numerical figures.
4. Think of the formula that best fits the question
5. Substitute your formula with the data available to you
6. Relate the variables from the formula with what you have underlined
7. Update your data
8. Solve the problem
10. Another formula we can apply to calculate threshold frequency involves the question that mentioned the work function of a metal which is f0 = w0/h [ where f0 = threshold frequency, w0 = work function of a metal, and h= Planck’s constant = 6.6×10-34Js
Solved Problems: How to Calculate the Threshold Frequency
Here are a few problems to guide you:
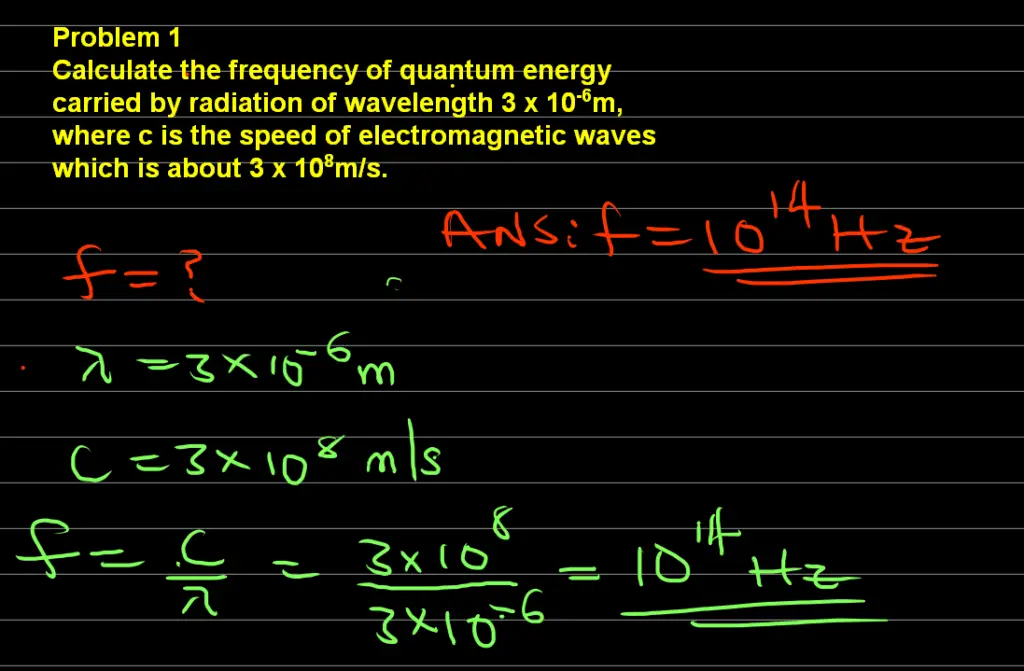
Problem 1
Calculate the frequency of quantum energy carried by radiation of wavelength 3 x 10-6m, where c is the speed of electromagnetic waves which is about 3 x 108m/s.
Solution
Data:
Wavelength of the radiation, λ = 3 x 10-6m
Speed of light in vacuum, c = 3 x 108m/s
Frequency, f = ?
since f = c/ λ
We can substitute our data into the above formula for frequency
f = (3 x 108) / (3 x 10-6) = 1014Hz
Therefore, the frequency of the quantum of energy is 1014Hz
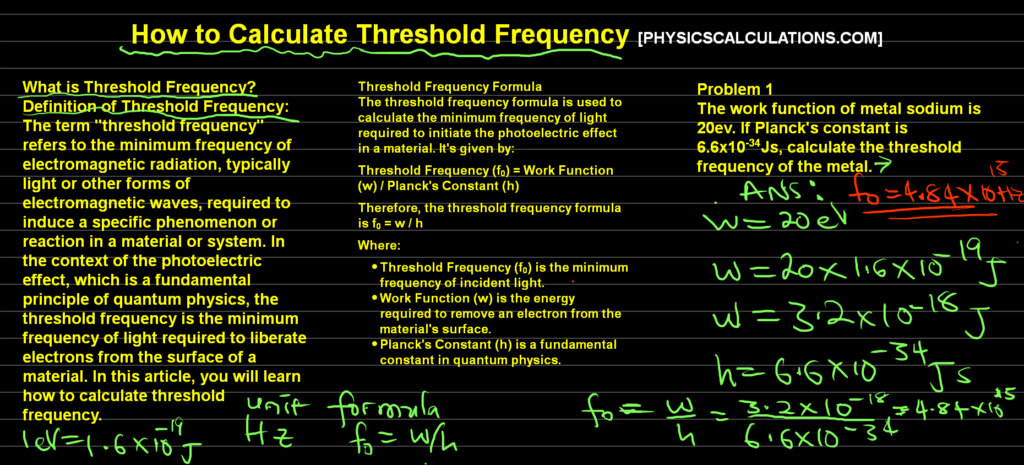
Problem 2
The work function of metal sodium is 20ev. If Planck’s constant is 6.6×10-34Js, calculate the threshold frequency of the metal.
Solution
Data:
The work function, w0 = 20ev = 3.2×10-18J
speed of light in vacuum, c = 3×108m/s
Planck’s constant, h = 6.6×10-34Js
f0 = ?
We substitute our formula f0 = w0/h to get
f0 = 3.2×10-18/6.6×10-34 = 4.84 x 1015 Hz
You may also like to read:
How to Calculate Power in a Circuit
How to Calculate the Kinetic Energy of an Ejected Electron
Reference