Question 1:
A photon with an energy of 1.33 x 10-21 Joules has a frequency of?
Answer
The answer to this question is 2.01 x 1012 Hz
Here is how I solved it:
Data
The first thing you will need to do is to extract your data from the above question. Here is the vital information we identified:
The energy of the photon, E = 1.33 x 10-21 Joules [ The unit of Energy of a photon is in Joules (J)]
Planck’s constant, h = Constant = 6.63 x 10-34 Joules-Second [ The unit of Planck’s constant is Joules-Second (J.s)]
The quantity to find:
Frequency, f = ?
The formula to apply:
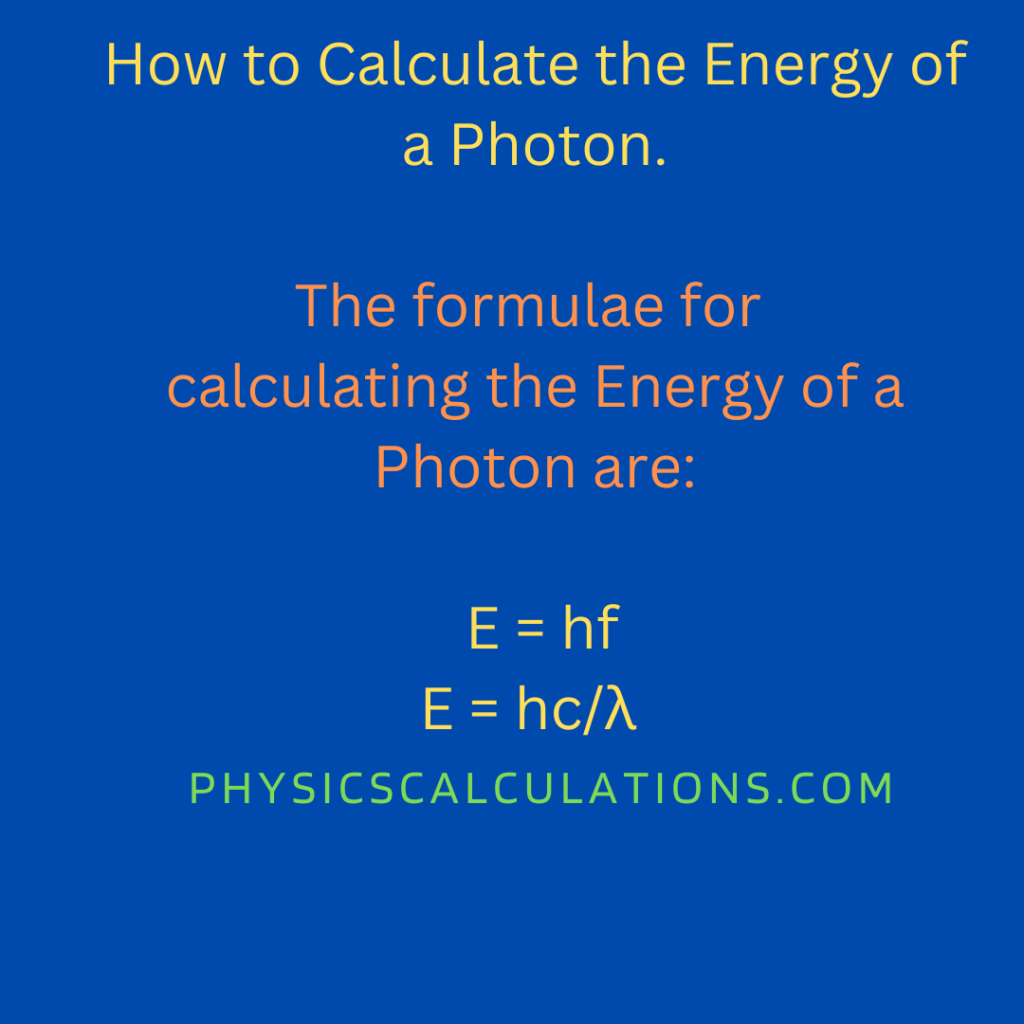
The formula to calculate the frequency of the photon is E = hf
Solution
From our formula, we have E = hf
But we are looking for the frequency, f
Hence, we make the f subject of the formula from the expression E = hf.
The first step is to divide both sides by h to obtain
f = E/h
We can then go ahead to substitute our values from our data into the above expression
f = 1.33 x 10-21 J / 6.63 x 10-34 J.s
You can see that for the units above, Joules (J) will cancel Joules (J) to leave us with per second (1/S or s-1 which is also the unit of frequency)
Therefore, we can write it as
f = (1.33 x 10-21 J / 6.63 x 10-34) s-1 [ we can change s-1 to Hz if we want to do so]
Now, apply a calculator to solve 1.33 x 10-21 J ÷ 6.63 x 10-34
Therefore, we will now get
f = 2,006,033,182,503.77 = 2.01 x 1012 s-1 or 2.01 x 1012 Hz.
Therefore, the photon with an energy of 1.33 x 10-21 Joules has a frequency of 2.01 x 1012 Hz
Question 2:
A photon with an energy of 1.33 x 10-21 Joules has a wavelength of?
Answer
The answer to this question is 1.5 x 10-5 meters.
Here is the proof:
Data: Identify your data from the question
The energy of the photon, E = 1.33 x 10-21 Joules [ The unit of Energy of a photon is in Joules (J)]
Planck’s constant, h = Constant = 6.63 x 10-34 Joules-Second [ The unit of Planck’s constant is Joules-Second (J.s)]
Speed of light, c = 3.0 x 108 m/s
The quantity to find:
The wavelength, λ =?
The formula to apply:
E = hc/λ
Here is how I arrived at the formula:
The formula to calculate the energy of a photon is E = hf, and frequency, f = c/λ
Hence, E = h x c/λ = hc/λ
Solution
Since E = hc/λ
We can make the λ subject of the formula from the above expression
After cross-multiplication
The formula for calculating wavelength, λ = hc/E [Note: The unit of wavelength of a photon is in meters (m)]
Now, insert your values from the data you obtained from the question into the above expression to get
Wavelength, λ = (6.63 x 10-34 x 3.0 x 108)/1.33 x 10-21
The above expression is now equal to
λ = (6.63 x 10-34 x 3.0 x 108)/1.33 x 10-21
Therefore, we will now get
λ = 1.989 x 10-25/1.33 x 10-21 = 0.00015 m or 1.5 x 10-5 m
Therefore, the photon with an energy of 1.33 x 10-21 Joules has a wavelength of 1.5 x 10-5 meters.
You can also calculate the energy of a photon by applying the energy of a photon calculator.
You may also like to read:
How to Calculate the Work Function of a Metal
Solved Problem: How to Calculate the Wavelength of Radiation
How to Calculate the Kinetic Energy of an Ejected Electron
And also
Calculate the Energy of a Photon of Each Frequency